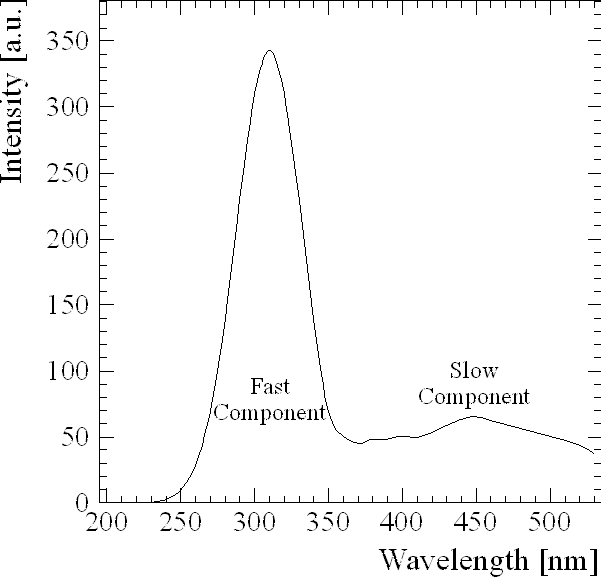







Interactions of ionizing radiation with matter lead (to a certain extent) to the excitation of electrons into higher energy states or to the creation of electron-hole pairs, respectively. The radiative deexcitation is characterized by the emission of photons whose wavelengths correspond to the energy of the excited state since E=h n . Only few materials show the emission of visible - or better: detectable - light, the so called luminescence. (NaI is capable to reemit 7% of the energy of the ionizing radiation as light [Bir64].) The number of generated photons in principle is proportional to the energy loss of an ionizing particle traversing a scintillating material. Important and widely used inorganic scintillators are alkalihalides like NaI, CsI and BaF that often are doped with activators (e.g. Thallium) in order to yield a high light output. Unfortunately these activators act as electron traps and thus delay the emission of light. Most fast scintillators on the other hand normally show low light output and emit light in the UV-region. For pure alkalihalides (of which NaI and CsI are the most common and best studied) there are three possible processes that can result in luminescence. One is caused by defects of the crystal structure that can act as traps for bound electron-hole pairs (excitons). The deexcitation, in general, happens within a few nanoseconds and can be non-radiative or radiative [Enz58]. The latter case is considered to be responsible for the fast decay component in the near UV. A competing effect resulting in fast scintillation light is the so called Crossluminescence [Jan87]. It is caused by radiative transitions of the electrons between valence band (5p)6 and upper core band (5p)5[Kub88]. But, having the valence band width (2.4±0.3 eV) subtracted from the difference of threshold energies (7.6±0.4 eV) [Smi75], the maximum wavelength for CsI crossluminescence would be at 240(+35) nm. However, Jansons et al. suspect Auger transitions to be responsible for the absence of a fast UV crossluminescence light in CsI [Jan87].
Another component emerges when electrons are temporary bound within vacancies; these vacancies presumably occur because of an content replacing -Ions during the growing process[Kub88]. Since this is a stronger metastable state it results in the slow emission of visible light that is responsible for the slow component. It was found by Hamada et al. [Ham95] that this component (in the case of CsI) slowly disappears with time and can be suppressed by moderate heat treatment, although there was a growing enhancement of the slow component for higher temperatures starting at 150°C. This way they could conclude that intrinsic vacancies are the source for the slow component. (Section 3.2.3 will be dedicated to the ratio of the slow luminescence component to the fast luminescence component.)





